Creating Safer, Smarter Medical Devices: Engineered to Cure
Table of contents
As our world population ages and medical science continues to advance, the demand for medical devices has never been higher. People are living longer, and medical technology plays a crucial role in improving their quality of life. But how do we ensure that these devices are not only safe and effective but also provide patients with the best possible experience?
These are the questions that medical designers and engineers grapple with daily. They strive to create devices that not only meet the highest standards of safety and effectiveness but also enhance the overall experience for patients.
In this industry, where time is of the essence, engineers are always seeking ways to streamline development and testing processes to bring devices to market faster. This is where virtual experimentation comes into play, offering a disruptive approach to medical device development.
Finding Better Medtech Solutions: Faster, Safer, Certified
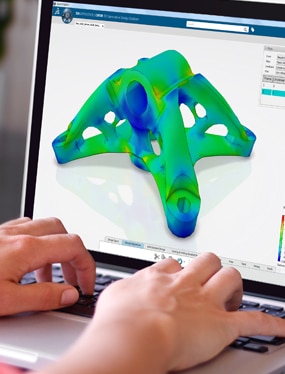
The Engineered to Cure Industry Solution Experience provides a virtual space where medical designers and engineers can experiment with promising device designs in real-world scenarios without the need for expensive and time-consuming physical testing on animals or humans.
This same virtual space also helps validate designs while ensuring compliance with regulatory requirements and business objectives. It even integrates the entire product development and certification business processes, offering full visibility and traceability.
Built on the 3DEXPERIENCE platform, Engineered to Cure embraces a data-driven, model-based engineering approach that enables teams to systematically collaborate on designing robust, safe, and personalized medical devices.
In this dynamic virtual environment, ideas can be tested and refined by multiple teams from different disciplines. The result? Safer, higher-performing, and more connected medical devices that can transform patient care.
The Changing Landscape of the Medtech Industry
The medical technology (medtech) industry has a rich history of patient-centered innovation. In 2022 alone, medtech companies invested over $42 billion in research and development (R&D). However, in recent times, the industry has faced unprecedented disruption and challenges, including high inflation, constrained capital markets, supply chain uncertainties, and geopolitical tensions. After a decade of robust performance, value creation in medtech has started to decelerate.
McKinsey’s recent report highlights critical findings that underscore the need for transformative strategies in the next decade. These findings resonate deeply with the Engineered to Cure solution offered by Dassault Systèmes, ushering in a new era of innovation and efficiency.
One of McKinsey’s key insights emphasizes the imperative for medtech companies to create value not only for their shareholders but, more importantly, for patients. The Engineered to Cure solution embodies this principle by enabling the streamlined development of medical devices that are safer, more effective, and genuinely patient-centered.
By integrating the entire product development and certification processes, companies can ensure full visibility and traceability, ensuring that the devices meet the highest standards of safety and compliance. This not only enhances patient outcomes but also represents a significant stride in value creation for medtech companies.
Moreover, the report underscores the importance of research and development excellence. Engineered to Cure offers a data-driven, model-based engineering approach that empowers R&D teams to innovate with unprecedented efficiency. With powerful modeling and simulation capabilities, including 3D design and virtual human modeling, teams can rapidly test and refine ideas in a collaborative virtual space. This aligns perfectly with the call for R&D excellence, as it accelerates innovation, reduces development costs, and ensures that the most robust and effective devices reach the market swiftly.
In Our Experience…
By incorporating software components and advanced simulation tools, Engineered to Cure fosters innovation and helps medtech companies to meet the challenges of tomorrow’s healthcare landscape head-on.
Whether it’s optimizing device performance, ensuring compliance with evolving regulatory standards, or enhancing patient experiences through software-driven solutions, Engineered to Cure equips medtech companies with the tools they need to thrive in the digital age.
In its essence, the medtech industry is driven by the desire to do well by doing good. With a clear vision, a passion for innovation and excellence, and a steady guiding hand, medtech leaders can chart their path to value creation, benefiting patients and stakeholders alike.
The future of medical device development lies in virtual experimentation, collaboration, and a commitment to making healthcare safer, more effective, and more patient-centered.